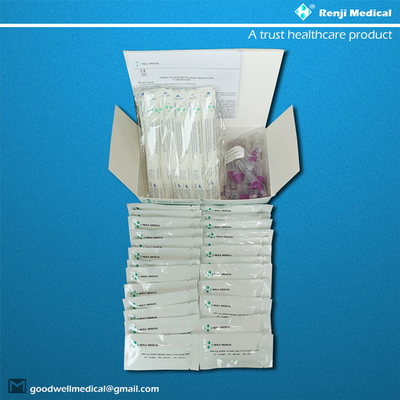
【PRODUCT NAME】
SARS-CoV-2 (COVID-19) Antigen Rapid Test Kit (Colloidal Gold)
【PACKAGE SPECIFICATION】
25 tests/kit & 50 tests/kit.

【INTENDED USE】
This kit is intended to be used for the qualitative detection of SARS-CoV-2 antigen in human
respiratory specimens, sputum, and other samples. This kit uses a cellulose membrane
immunochromatography technology.
Antigen detection is used for auxiliary diagnosis or epidemiological investigation of human
infection with SARS-CoV-2.
The COVID-19 Antigen Rapid Test Device is a rapid visual immunoassay
for the qualitative, presumptive detection of COVID-19 antigens from throat
swabs and nasopharyngeal swab specimens. The test is intended for use
as an aid in the rapid differential diagnosis of acute COVID-19 virus
infection.
【When should I use a rapid antigen test?】
If you are a close contact, you can use a rapid antigen test for your initial and day
6 test. You can also purchase and use rapid antigen tests from us:
- before entering a high risk setting, e.g. healthcare or aged care
- before going out where there may be crowds
- before going to work, especially if it is a critical worksite
- to provide reassurance if you have no symptoms but are feeling anxious
or worried.
【When should I get a standard PCR test?】
You should get a PCR test as soon as you develop any COVID-19 symptoms and
are not a close contact.
COVID-19 symptoms include:
• fever (a temperature of 37.5˚C or higher) or chills
• cough
• loss of taste or smell
• sore throat
• tiredness (fatigue)
• runny or blocked nose
• shortness of breath (difficulty breathing)
• nausea, vomiting or diarrhoea
• headache
• muscle or joint pain
• loss of appetite
You will also need to get a PCR test if you have COVID-19 symptoms and test
negative using a rapid antigen test to confirm your result.
Accessing rapid antigen tests
If you are not a close contact, rapid antigen tests are sold commercially in
supermarkets and pharmacies.
【 What are antigens?】
An antigen is a molecule capable of stimulating an immune response.
They may be proteins, polysaccharides, lipids, or nucleic acids.
Each antigen has distinct surface features that are recognized by the immune system.
SARS-CoV-2, the virus that causes COVID-19, has several known antigens,
including its nucleocapsid phosphoprotein and spike glycoprotein, which are the
visible protrusions on its surface.
【What are antigen tests and what can they tell us?】
An antigen test reveals if a person is currently infected with a pathogen such as
the SARS-CoV-2 virus. Once the infection has gone, the antigen disappears.
Unlike nucleic acid based tests such as PCR, which detect the presence of genetic
material, antigen tests detect proteins or glycans, such as the spike proteins found
on the surface of the SARS-CoV-2 virus.
They can take longer to develop than molecular and antibody tests, as suitable
antibodies for use in the assays must first be identified and produced, which can
be a complex and time-consuming process. Accuracy can also be a problem, with
antigen tests typically having a much lower sensitivity than PCR.
However, they usually provide test results rapidly, are relatively cheap, and can be
more amenable to point-of-care use, which could make them more suitable for
testing in the community and in remote regions.
【What's the difference between a PCR and Antigen Covid-19 test?】
All COVID-19 tests start with a sample, but the scientific process goes very differently
after that.
At this point in the pandemic, you or someone you know has probably received
at least one COVID-19 test. But do you know which kind of test you got and the
strengths and weaknesses of these different tests?
Two major types of tests are used to diagnose infection with SARS-CoV-2:
molecular tests – better known as PCR tests – and antigen tests. Each detects a
different part of the virus, and how it works influences the test’s speed and relative
accuracy. So, what are the differences between these types of tests?
PCR tests are extremely accurate but require special lab equipment – like the PCR
heating machine seen here – and can take hours or days to perform.
Looking for genetic evidence
The first step for either kind of test is to get a sample from the patient. This can be
a nasal swab or a bit of saliva.
For PCR tests, the next step is amplification of genetic material so that even a small
amount of coronavirus genes in the patient’s sample can be detected. This is done
using a technique called a polymerase chain reaction. A health care worker takes
the sample and treats it with an enzyme that converts RNA into
double-stranded DNA. Then, the DNA is mixed with a solution containing an
enzyme called a polymerase and heated, causing the DNA to separate into two
single-stranded DNA pieces. The temperature is lowered, and polymerase, with the
help of a small piece of guide DNA called a primer, binds to the single-stranded
DNA and copies it. The primers ensure that only coronavirus DNA is amplified.
You’ve now created two copies of coronavirus DNA from the original one piece of RNA.
Laboratory machines repeat these heating and cooling cycles 30 to 40 times, doubling
the DNA until there are a billion copies of the original piece. The amplified sequence
contains fluorescent dye that is read by a machine.
The amplifying property of PCR allows the test to successfully detect even the smallest
amount of coronavirus genetic material in a sample. This makes it a highly sensitive
and accurate test. With accuracy that approaches 100%, it is the gold standard for
diagnosing SARS–CoV–2.
However, PCR tests have some weaknesses too. They require a skilled laboratory
technician and special equipment to run them, and the amplification process can
take an hour or more from start to finish. Usually only large, centralized testing
facilities – like hospital labs – can conduct many PCR tests at a time. Between sample
collection, transportation, amplification, detection and reporting, it can take from
12 hours to five days for a person to get results back. And finally, they aren’t cheap
at $100 or more per test.
Rapid Antigen tests
Rapid, accurate tests are essential to contain a highly contagious virus like SARS-CoV-2.
PCR tests are accurate but can take a long time to produce results. Antigen tests,
the other major type of coronavirus test, while much faster, are less accurate.
Antigens are substances that cause the body to produce an immune response –
they trigger the generation of antibodies. These tests use lab-made antibodies to
search for antigens from the SARS-CoV-2 virus.
To run an antigen test, you first treat a sample with a liquid containing salt and soap
that breaks apart cells and other particles. Then you apply this liquid to a test strip
that has antibodies specific to SARS-CoV-2 painted on it in a thin line.
Just like antibodies in your body, the ones on the test strip will bind to any antigen
in the sample. If the antibodies bind to coronavirus antigens, a colored line appears
on the test strip indicating the presence of SARS-CoV-2.
Antigen tests have a number of strengths. First, they are so easy to use that people
with no special training can perform them and interpret the results – even at home.
They also produce results quickly, typically in less than 15 minutes. Another benefit
is that these tests can be relatively inexpensive at around $10-$15 per test.
Antigen tests do have some drawbacks. Depending on the situation, they can be
less accurate than PCR tests. When a person is symptomatic or has a lot of virus in
their system, antigen tests are very accurate. However, unlike molecular PCR tests,
antigen tests don’t amplify the thing they are looking for. This means there needs to
be enough viral antigen in the sample for the antibodies on the test strip to generate
a signal. When a person is in the early stages of infection, not a lot of virus is in the
nose and throat, from which the samples are taken. So, antigen tests can miss early
cases of COVID-19. It’s also during this stage that a person has no symptoms, so
they are more likely to be unaware they’re infected.
Understanding the strengths and limitations of both PCR and antigen tests, and
when to use them, can help to bring the COVID-19 pandemic under control.
So, the next time you get a COVID-19 test, choose the one that is right for you.
COVID-19 RAPID ANTIGEN TEST
The COVID-19 rapid antigen test detects protein fragments specific to the
Coronavirus. Rapid antigen testing is a faster way to detect if you have a current
COVID-19 infection. Rapid antigen testing may be helpful for those who want
quick results to meet a testing requirement for an event or travel. For example,
this test can be used if you plan to visit a location or establishment that requires
testing prior to arrival. Testing requirements vary by location and may change
over time. This test requires a nasal or nasopharyngeal swab. With our product
testing, results may come back in as soon as 10 – 15 minutes.
Common COVID-19 symptoms include fever, cough, and shortness of breath.
How the test works?
A sample is collected through a nasal or throat swab and mixed with
a solution. The solution is then placed onto an indicator device that may
detect the presence of the virus that causes COVID-19.
Test Purpose: Used to detect an active infection.
Sample Type: Nasal or Nasopharyngeal Swab
Results Time: 10 – 15 Minutes*
(Wait times may vary depending on volume)
All COVID-19 tests start with a sample, but the scientific process goes very
differently after that.
At this point in the pandemic, you or someone you know has probably received
at least one COVID-19 test. But do you know which kind of test you got and the
strengths and weaknesses of these different tests?
Two major types of tests are used to diagnose infection with SARS-CoV-2:
molecular tests – better known as PCR tests – and antigen tests. Each detects
a different part of the virus, and how it works influences the test’s speed and
relative accuracy. So, what are the differences between these types of tests?
PCR tests are extremely accurate but require special lab equipment – like the PCR
heating machine seen here – and can take hours or days to perform.
Rapid antigen tests offer a quick and easy way to screen for COVID-19 at home.
In about 15 minutes, they detect active infections via a nasal swab, including in
asymptomatic individuals. Often called “rapid tests” or “at-home COVID tests,”
these rapid antigen tests can be a valuable tool for managing life during the pandemic
—if you can get your hands on one.
Rapid antigen tests are used as a test to diagnose COVID-19.
This means that if you test positive using a rapid antigen test, your result
does not need to be confirmed with a PCR test.
In only 15 minutes, you will have the results you need to confidently return to work,
school, sports and all the things you love to do, with the ability to store and share
our family's and your test results from Renji Antigen Rapid Test.
With Renji COVID-19 Antigen Rapid Test, you can test yourself after being out in
public. Early and regular testing helps you better care for yourself and protects
your friends, family, and community members from potential exposure.
【PRINCIPLE】
The kit adopts cellulose membrane immunochromatography technology and applies the
principle of membrane chromatography double antibody sandwich method to detect the
SARS-CoV-2 antigens in human samples. The product consists of a sample well and detection
the area on the Detection Strip. The Detection strip contains coated anti-SARS-CoV-2 monoclonal
antibody, colloidal gold particles labeled with anti-SARS-CoV-2 a monoclonal antibody, and
goat anti-mouse polyclonal antibody. When SARS-CoV-2 antigens are present in the sample,
the SARS-CoV-2 antigens will combine with the colloidal gold-labeled anti-SARS-CoV-2
a monoclonal antibody will be captured by the coated anti-SARS-CoV-2 monoclonal antibody.
When the viral antigen crosses the T region of the detection line, it will be captured by the
coated anti-coronavirus. A monoclonal antibody will gather and develop color in the detection
area. Conversely, if SARS-CoV-2 antigens are not present in the sample or the antigen titer
is below the limit of detection, there will be no color band appearance on the detection line and
the result will be negative. Regardless of whether the sample contains antigens of the
SARS-CoV-2, a color band should appear on the quality control region (C region) line of the
detection area. The quality control band is the standard for judging whether the inspection
process is normal and it also serves as the internal control standard for the Detection Strip.
【KIT COMPONENTS】
| Components |
25T |
50T |
- SARS-CoV-2 test card
|
25 pcs |
50 pcs |
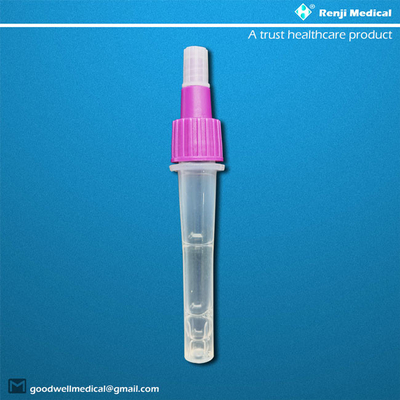
- Disposable sampling tube (with sample diluent buffer)
|
25 pcs |
50 pcs |
- Disposable sampler
|
25 pcs |
50 pcs |
- Instructions
|
1 copy |
1 copy |
| Note: The components in the kits of different batch numbers are not interchangeable. |
The materials and instruments necessary for the test but not provided are as follows:
Lancet;
Absorbent paper or similar material;
Timer;
Micropipette corresponding to the range;
Laboratory safety protection equipment such as disposable gloves, etc.
【STORAGE CONDITIONS AND SHELF LIFE】
Stored at 2°C ~ 30°C, and kept away from direct sunlight.
The validity period is 12 months.
After the aluminum foil bag is opened, the Detection Strip should be used within 1 hour.
The Sample Diluent Buffer should be capped immediately after usage.
Usage after the date of expiration is not recommended.
The date of manufacture & expiration is indicated on the label and package.
【SAMPLE REQUIREMENTS】
Sample type: nasal swabs, throat swabs, deep cough sputum, respiratory tract extracts,
bronchial lavage fluid, alveolar lavage fluid, etc.
Sample collection: the collection and testing of patient samples must be performed in accordance
with the “Guidelines on Laboratory Testing Techniques for COVID-19 Caused by SARS-CoV-2”
(The 4th edition) released by the National Health Commission.
Sample processing:
1. Take 500 µL (deep cough sputum, respiratory tract extract, bronchial lavage fluid, alveolar
lavage fluid) from sample to be tested, mix it with 500 µL of sample diluent solution (1:1), and
then take 100 µL into the sample well for testing;
2. Samples such as nasal swabs or throat swabs are directly put into 500 µL sample diluent
solution, mixed thoroughly, and then about 100 µL of the supernatant is taken for testing.
Sample storage: the samples should be processed for testing in time after collection; if not,
they should be stored in accordance with the requirements of the “Guidelines on Laboratory
Testing Techniques for COVID-19 Caused by SARS-CoV-2” (The 4th edition) released by the
National Health Commission.
Sample safety: all samples should be treated as potentially infectious materials and subject to
the relevant standards and guidelines.
【EXPERIMENT WORKFLOW】
Please read the instruction for use carefully before using this kit. All reagents should be
incubated at room temperature (10-30°C) for 30 minutes prior to use. The test should be
carried out at room temperature and the operation procedure is described below:
1. Open the sealed bag and remove the Detection Strip. Mark the sample ID on the test strip
and lay the strip flat on the table.
2. Specimen collection
1). Carefully insert the swab into the nostril of the patient, reaching the surface of the posterior
nasopharynx, that presents the most secretion under visual inspection.
2). Swab over the surface of the posterior nasopharynx. Rotate the swab several times.
3). Withdraw the swab from the nasal cavity.

3. Sample preparation
1). Insert the sample diluent tube into the pipe rack, make sure that the tube is standing firm
and reaches the bottom of the pipe rack.
2). Open the purple cap of the sample diluent tube. Insert the swab into the diluent tube which
contains 0.5 mL of the diluent buffer. Roll the swab at least 6 times while pressing the head
against the bottom and side of the diluent tube.
3). Leave the swab in the diluent tube for 1 minute.
4). Squeeze the tube several times with fingers from outside of the tube to immerse the swab.
Remove the swab, close the cap. The diluent solution will be used as test sample.
5). Open the small cap on the top of the sample dilution tube. Add 3-4 drops (~100 μL) of the
sample diluent buffer immediately to the sample well.
6). Allow the strip to develop for 10-15 minutes at room temperature. A visible band can be
read by naked eyes.


【RESULTS ANALYSIS】
The interpretation of visual interpretation results (as shown below):
1. The results can be read directly by naked eyes as shown in the figures below: Positive result:
a visible band can be seen in both line C and line T.

2. Negative result: A visible band can be seen in line C only.
3. Invalid result: A visible band cannot be seen in the line C, and the test must be repeated
using a new strip.
【MANAGEMENT OF RESULTS】

【REPORTING YOUR RESULT】
You are legally required to report all positive rapid antigen test results. You must
report your positive result if you get your test from a designated RAT Collection
Point, from a retail outlet, from your workplace or through any other means
(i.e. over the internet).
You can also report negative and invalid test results to help provide a full picture
of rates of COVID-19 testing in the state.
【WHAT DO I DO IF I HAVE A POSITIVE RESULT?】
If you test positive in a rapid antigen test, this is considered a case. You do not need
to get a PCR test to confirm your result.
You must:
• report your result as soon as possible
• isolate for 10 days from the day you had a positive result
• notify your close contacts that you have tested positive.
Read more information about what to do if you test positive to COVID-19.
If you are a close contact with no COVID-19 symptoms and test positive in your day
6 test, you are able to get a PCR test to confirm your result.
【WHAT DO I DO IF I HAVE A NEGATIVE RESULT?】
If you are close contact with no COVID-19 symptoms and test negative, you do not
need to get a PCR test to confirm your result.
If you have COVID-19 symptoms and test negative, you must get a PCR test to
confirm your result. Rapid antigen tests are not as sensitive as PCR tests, which
means that just because you have a negative result in a rapid antigen test, it does
not mean that you do not have COVID-19.
【CUT-OFF VALUE】
1. Determination of the cut-off value, the product is a qualitative detection kit of antigens to
the SARS-CoV-2. When the quality control line (C line) and the test line (T line) are formed,
regardless of a weak T line, as long as it is visible to the normal naked eyes, the test result
should be judged as positive.
2. The test for the normal population should be negative. Positive test results indicate that an
individual may have been exposed to SARS-CoV-2, and should be combined with clinical
symptoms and other diagnostic results for further confirmation.
【INTERPRETATION OF TEST RESULTS】
1. Common mistakes that may lead to false-positive or negative results include: the kit is used
after its expiration; the kit has been improperly stored; the operating temperature is too low
(<4°C) or too high (>30°C); the procedures outlined in this protocol were not strictly adhered to.
2. Any final clinical interpretation should consider a combination of test results, clinical
symptoms, and other indicators.
【LIMITATIONS OF INSPECTION METHODS】
1. If the patient has clinical symptoms but the test result is negative, it is recommended to use
the PCR method for confirmation and the doctor will make a comprehensive judgment to
confirm the diagnosis. The negative result cannot be the only evidence to rule out the
SARS-CoV-2 infection.
2. The product can only be used for clinical diagnosis and on-site screening of the SARS-CoV-2.
The positive results of all Detection Strips must be confirmed by a qualified laboratory. The
positive result of antigen detection should be combined with other clinical features to ensure
an accurate diagnosis.
3. In order to ensure the accuracy of the SARS-CoV-2 antigens detection, high temperature
and humidity must be avoided.
【PERFORMANCE CHARACTERISTICS】
1. Coincidence rate of negative reference: test 15 SARS-CoV-2 antigen-negative references with
the kit. The coincidence rate of 15 negative references should be 100%.
2. Coincidence rate of positive reference: test 5 SARS-CoV-2 antigen-positive references with
the kit. The coincidence rate of 5 positive references should be 100%.
3. Limit of detection (LOD): test 3 LOD references with the kit. S1 can be positive or negative
and S2~S3 should be positive.
4. Reproducibility: test 2 repeatable references R1 and R2 for 10 times with the kit, the results
should be all negative for R1 and positive for R2.
5. Inter-run variability: test 2 repeatable references R1 and R2 10 times with 3 batches of kits,
the results should be consistently and all negative for R1 and positive for R2.
【PRECAUTIONS】
1. This product is a disposable in-vitro diagnostic reagent. Do not reuse. Do not use this kit if
it is expired.
2. In order to obtain the correct test results, the test should be operated strictly according to
the Instruction for Use.
3. The Detection Strips and Sample Diluent Buffer should be brought up to room temperature
before use.
4. All test samples and waste should be treated as infectious and disposed of in accordance
with local regulations.
5. The kit should be stored at 4-30℃.Do not freeze, avoid heavy pressure during storage and
keep away from moisture, light, and heat.
6. When transporting at room temperature, light handling, light loading, and unloading,
avoiding heavy pressure, waterproofing, and high-temperature resistance must be observed
during transportation.
7. It is recommended to use fresh samples instead of repeated freeze-thaw samples. Pay
attention to safety measures during operation, such as wearing protective clothing and gloves.
8. Desiccant is contained in the aluminum foil bag. Do not ingest.
【STORAGE CONDITIONS AND SHELF LIFE】
Stored at 2°C ~ 30°C, and kept away from direct sunlight.
The validity period is 12 months.
After the aluminum foil bag is opened, the Detection Strip should be used within 1 hour.
The Sample Diluent Buffer should be capped immediately after usage.
Usage after the date of expiration is not recommended.
The date of manufacture & expiration is indicated on the label and package.
【INDEX OF SYMBOL】

【EXPORTER】
Magnus International Limited
F12, New City International Mansion A, 234 Huapao Ave.
Liuyang, Hunan Province 410300 China
Contact: Goodwellmedical@gmail.com
【MANUFACTURER】
Changsha Renji Medical Equipment Co., Ltd.
No.18 Xiangtai Road, Liuyang Jingkai District,
Changsha City, Hunan Province 410300 China
【AUTHORIZED REPRESENTATIVE】
Lotus NL B.V.
Koningin Julianaplein 10, 1e Verd, 2595AA, The Hague, Netherlands.
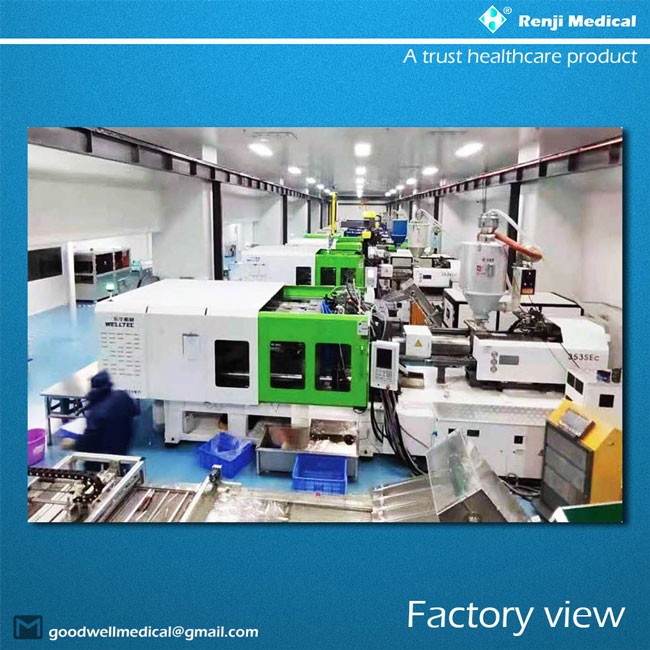
【FACTORY VIEW】


【FACTORY WORKSHOP】

【PRODUCT AND STANDARDS】

【CERTIFICATIONS】


【FAQ】
1. Q: How about your healthcare and medical product quality?
A: Quality is our top priority. We have a professional quality control team to ensure the
quality with a high standard and regulated with the pro certifications. The QC team is
responsible for quality checking in each process and inspect every batch of our production
and ship them only after QC approval.
2. Q: What is your order MOQ?
A: Please contact the customer service, and the price is negotiable for large quantity.
3. Q: How about your product packing for shipment or mailing?
A: We prepared well neutral package for our products. We could also make OEM package,
OEM/ODM Custom Package/Multi-packing package/Cartons with well protective wrapping.
4. Q: What level of your product price?
A: We are the factory and direct manufacturer and offer competitive prices. We also offer
the volume pricing with more benefits depend on your order quantity level. Please let us know
your ordering volume while you make the inquiry to us to help you obtaining better benefits.
5. Q: What's the price terms?
A: The price is based on FOB of Hunan, China with exchange rate at RMB6.40 for USD$1.00.
If the exchange rate floating sharply lower than 6.3 or higher than 6.5, we should adjust the
price with real-time exchange rate.
6. Q: What's the payment terms?
A: We accept T/T wire transferring, PayPal, Western Union, etc. 50% down payment after
signing the contract and rest 50% before shipping confirmation.
7. Q: How could I get some samples?
A: We are able to deduct your samples expense once your orders confirmed with us.
The shipping fee will be calculated with the sample volume what you demand.
8. Q: Could you accept OEM order?
A: Yes, we could. We have professional designers who can design the logo or artwork and offer
best solution & styles for your OEM product choices.
9. Q: How long will the shipping or express take?
A: (1) For the international expresses such as DHL, UPS, FedEx, TNT, or EMS, etc., it usually
takes 5-7 working days to your location or destination.
(2) If shipping with containers, they will take about 30-60 days to process depending on
the vary ports or door locations.
(3) We will arrange your products delivery or shipping within 1-3 days after full payment
has been confirmed. We may not ship your products out until your payments arrive to
our bank account successfully.
10. Q: How do you deliver the goods to us?
A: If the order of small volume package, we could deliver by courier such as DHL, UPS, FedEx,
TNT, EMS. For the large volume order, we could ship with bulk cargo containers by sea.
We will provide you cost-saving recommendations for your product shipping.
11. Q: What is the warranty time.
A: One year f each product.
【SHIPPING】
We are the trusted healthcare and medical supplies from China.
Be free to contact us at any time!
You are very welcome to email us today at:
Goodwellmedical@gmail.com for the inquiry!
We are excited to build a reliable and long-term business with you
at our best!
 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!