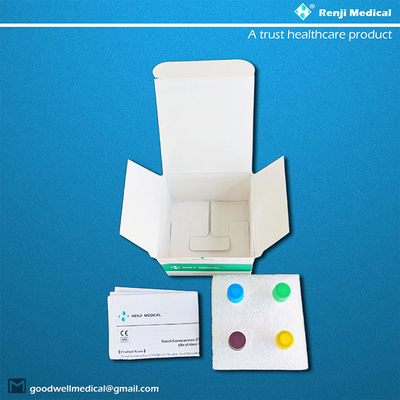
【Product Name】
Novel Coronavirus (COVID-19) Nucleic Acid Detection Kit
(Real-time RT-PCR Method)
【Package Specification】
50 tests/kit & 200 tests/kit

【Intended Use】
This kit is used for the detection of COVID-19(ORF1ab/N Gene) Nucleic Acid and
assisted diagnosis and epidemiological surveillance of COVID-19. This is a
qualitative PCR test for the detection of nucleic acid in nasopharyngeal swabs
from patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Patients may have mild to severe respiratory illness with symptoms of sore throat,
cough, fever and shortness of breath and many have had complications including
pneumonia. Patients may also be asymptomatic individuals with recent known or
suspected exposure to SARS-CoV-2 or asymptomatic individuals without known
or suspected exposure to SARS-CoV-2 for early identification in special settings.
【Test Principle】
The kit adopts the principle of real-time fluorescent quantitative PCR technology,
designs specific primers and probes for COVID-19(ORF1ab/N gene) ,
and combines them with real-time fluorescent quantitative PCR instrument to
detect the nucleic acid of COVID-19 virus. So as to realize the qualitative detection
of COVID-19 virus nucleic acid.
In addition, the PCR detection system uses the positive internal control,
which monitors the presence of PCR inhibitors in test specimens by detecting
whether the internal control signal is normal,to avoid a false negative result.
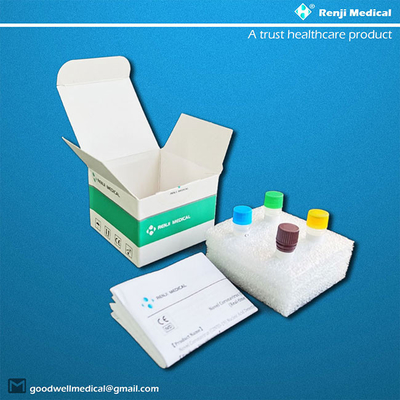
【Components of the Diagnostic Kit】
| No. |
Reagent Name |
Spec. & Qty. |
|
| |
|
50T |
200T |
| 1 |
COVID-19 PCR Mix |
750µl x 1 tube |
750µl x 4 tube |
| 2 |
COVID-19 Enzyme Mix |
250µl x 1 tube |
250µl x 4 tube |
| 3 |
COVID-19 Negative Control |
100µl x 1 tube |
100µl x 4 tube |
| 4 |
COVID-19 Positive Control |
100µl x1tube |
100µl x4tube |
| 5 |
Instructions |
1 serving |
1 serving |
Note:
1. Do not mix or exchange components from different kit lots.
2. The COVID-19 Negative Control is sterile nuclease-free water, and the COVID-19
Positive Control is transcribed RNA invitro which contains target genes ORF1ab,
N gene, and Internal Control gene.
【Applicable Instrument】
The diagnostic kit is applicable to MA-6000,ABI series, Bio-Rad series, Roche
LightCycler R480, Cepheid SmartCycler, Rotor-Gene series and other multi-channel
real-time quantitative PCR instruments.
【Specimen Requirements】
1. Sample types:Upper respiratory tract specimens(including throat swabs,
nasal swabs, nasopharyngeal extracts deep cough sputum), lower respiratory tract
specimens(including respiratory tract extracts, bronchial lavage fluid alveolar lavage
fluid, lung tissue biopsy specimens) , Tissue culture and other samples.
2.Storage conditions:The collected specimens should be submitted for inspection
in a timely manner, and the specimens should be stored at 4℃ within 24 hours.
It is best to store at-70℃ for more than 24 hours, and avoid repeated freeze-thaw
cycles.
【Storage and Stability】
1. The diagnostic kit should be stored in a sealed pouch at-20±5℃.The expiry date
is 12 months.
2. Please refer to the date of manufacture and expiry date on the outer package.
3. The reagent keep valid and stable within the expiry date if not used. The kit should
not be frozen and more than 5 times.
【Test Method】
1.Reagent Preparation (performed at “reagent preparation region”)
1.1 Takeout each component from the diagnostic kit and place them at room temperature.
Allow the reagents to equilibrate at room temperature, then vortex each of them
respectively for later use.
1.2 According to the quantity of test specimens, COVID-19 Positive Control and
COVID-19 Negative Control,pipette appropriate quantity of COVID-19 PCR Mix
and COVID-19 Enzyme Mix (COVID-19 PCR Mix 15 µl/test+COVID-19 Enzyme
Mix 5 µl /test) mix them thoroughly to make a PCR-Master mix centrifuge it
instantaneously for later use.
| Reagent name |
1 Sample |
10 Samples |
25 Samples |
50 Samples |
100 Samples |
200 Samples |
| COVID-19PCR Mix(µl) |
15 |
150 |
375 |
750 |
1500 |
3000 |
|
COVID-19 Enzyme
Mix(µl)
|
5 |
50 |
125 |
250 |
500 |
1000 |
|
PCR-Mastermix
|
20 |
200 |
500 |
1000 |
2000 |
4000 |
| Note:The above configuration is just you’re your reference and to ensure enough volume of the PCR-Mastermix, more volume of the actual pipetting may be required. |
1.3 Transfer the above-prepared reagents to the "specimen processing region" for
later use.
2.Processing and loading of specimens (performed at "specimen
processing region")
2.1 This diagnostic kit does not include Viral RNA&DNA Extraction Kit. It is
recommended to use Viral RNA&DNA Extraction Kit produced by Changsha Renji
Medical Equipment Co, Ltd. to extract viral RNA. The specific operation is in
accordance with its instructions.
2.2 Add 20 µl PCR-Mastermix into PCR reaction tube with 5µl above processed sample,
COVID-19 Positive Control and COVID-19 Negative Control and cap the tube. Carry
out fluorescence quantitative PCR detection on fluorescence PCR instrument.
3.PCR Amplification (performed at "nucleic acid amplification area")
3.1 Place PCR reaction tubes into the specimen wells of the amplification equipment
Setup COVID-19 Positive Control COVID-19 Negative Control and specimens to be
tested in the corresponding sequence and input specimen name.
3.2 Set cycle parameters according to the following table for PCR amplification.
| Steps |
Temperature |
Time |
Cycles |
| 1 |
50°C |
10min |
1 |
| 2 |
95°C |
3min |
1 |
| 3 |
95°C |
10s |
40 |
| 55°C |
30s |
Note:
1) The fluorescence collection is set at "Step3:55°C, 30s". Selection of detection
channels:FAM, HEX and Cy5, where FAM channel is ORF1ab gene and HEX
channel is N gene. Cy5 channel is the internal control gene, and the reaction system
s set to 25 µl.
2) ABI series fluorescent PCR instruments do not select ROX calibration and select
None for the quenching group.
【Positive Judgement Value and Reference Interval】
1. Condition Setting for Result Analysis
The adjustment principle of Baseline and Threshold is generally based on the results
of the automatic analysis of the instrument. When the overall slope of the curve
appears, the Start, End,and Threshold values of the Baseline can be adjusted
according to the image. Usually, the user can adjust it according to the actual
situation. The Start value can be set to 3-15, and the End value can be set to 5-20.
Adjust the amplification curve of the negative control to make it straight or below
the thresh hold line.
2. The Validity of The Kit
2.1 The COVID-19 Positive Control:FAM, HEX and Cy 5 channels have typical
S-type amplification curves and Cts≤32.
2.2 The COVID-19 Negative Control:FAM, HEX and Cy 5 channels have no
Ct or Ct>38.
2.3 Note:The above conditions must be met at the same time, otherwise this
experiment is invalid and needs to be repeated.
3.The Positive Judgment Value
Through the study of reference values, it was determined that the Ct reference value
of the target gene and the internal control gene detected by this kit was both 38.

【The Sample Result Judgment】
1.If the test sample detects atypical S-type amplification curve in the FAM, HEX
and Cy 5 channels and the Ct is≤38, the sample can be judged to be COVID-19
positive.
2.If the test sample has no Ct or Ct>38 in the FAM and HEX channels, and there
is atypical S-type amplification curve in the Internal Control channel(Cy 5) , Cts≤38,
the sample can be judged to be COVID-19 negative.
3.If the test sample only has a Ct values 38 in a single channel of the FAM or HEX
channel, and there is atypical S-type amplification curve in the Internal Control
channel(Cy 5) , Ct≤38, the results need to retest. If the results repeated are
consistent the sample can be judged as COVID-19 positive, the results repeated
are negative except for the typical S-type amplification curve of the Internal Control
channel(Cy 5) , Ct≤38, which could be judged as COVID-19 negative.
4.If no typical S-type amplification curve(No Ct value) or Ct value>38 is detected in
the FAM, HEX, and Cy 5 channels of the test sample, it means that there is a
problem with the quality of the sample or a problem with the operation. If the result
is invalid, you should find and eliminate the cause, collect the sample again,
and repeat the test(if the test result is still invalid, please contact the company) .
【Limitations of Detection Method】
1.Test results of the diagnostic kit can be used only for clinic reference. The clinical
diagnosis and treatment of patients should be considered in conjunction with their
symptoms, signs, medical history and other related conditions.
2.False negative results may occur when the concentration of the detected nucleic
acid in the test sample is below the minimum detection limit of this kit.
3.Improper handling of the tested sample during collection, transportation,
storage, and processing can easily result in RNA degradation and false negative
results.
4.When samples are cross-contaminated during collection, transportation, storage,
and processing, it is easy to get false positive results.
【Product Performance Index】
1.LOD:The limit of detection is 200 copies/ml.
2.Precision:Coefficient of variation (CV%] of precision Ct value within batch≤3%.
3.Specificity:There is no cross reaction between the kit and positive samples, such
as Human Coronavirus HCoV-NL63, Human Coronavirus HCoV-OC43, SARS
Coronavirus, MERS Coronavirus, Influenza A Virus, Influenza B Virus Yamagata,
Victoria, H1N1 Influenza Virus, H3N2 Influenza Virus, H5N1 Influenza Virus,
H7N9 Influenza Virus, Respiratory Syncytial Virus A, Adenovirus(type 2) ,
Adenovirus(type 2) , Mycoplasma Pneumoniae, Chlamydia Pneumoniae,
Pertussis, Streptococcus Pneumoniae, Rhinovirus(Type A) etc.
【Precautions】
1.The entire detection process should be performed strictly in accordance with the
requirements of this manual in the reagent preparation area, sample processing area,
and PCR amplification area, and the experimental clothes, instruments,
and consumables in each area should be used independently and cannot be mixed.
2.To avoid RNA degradation, the sample processing process should be operated at
0-4℃, and the test should be performed immediately after the experiment is
completed. Utensil consumables used in sample processing should be nuclease-free.
3.Negative and positive controls should be set for each experiment.
4.All reagents in the kit should be fully thawed and mixed at room temperature and
centrifuged immediately before use.
5.All negative and positive controls in the kit should be transferred to the sample
preparation area and stored separately before the first use.
6.To prevent fluorescence interference, avoid touching the PCR reaction tube
directly with bare hands, and avoid any marking on the PCR reaction tube.
7.The instrument amplification related parameters should be set in accordance with
the relevant requirements of this manual and different batches of reagents cannot
be mixed.
8.The product waste during the experiment should be detoxified before being
discarded.
【What is Nucleic Acid?】
Nucleic acids are essential for all forms of life, and it is found in all cells. Nucleic acids
come in two natural forms called deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
Nucleic acids are made of biopolymers, which are naturally-occurring, repeated sets of
monomers (making polymers) that then create nucleotides, which form nucleic acids.
To understand the structure of nucleic acid, it is important to understand the structure
of the nucleotides that make up nucleic acid.
Nucleic acid is an essential part of all living things and is the building block for both
DNA and RNA. It is found in all cells and also in some viruses. Nucleic acids have a
very diverse set of functions, such as cell creation, the storage and processing of
genetic information, protein building, and the generation of energy cells.
Although their functions may differ, the structures of DNA and RNA are very similar,
with only a few fundamental differences in their molecular make-up
differentiating them.
Sample Types: Sample types include anterior nasal, oropharyngeal, and
nasopharyngeal swabs for samples from asymptomatic individuals and individuals
suspected of having COVID-19 or asymptomatic individuals and saliva samples
collected using the Renji Saliva Collection Kit from individuals suspected
of having COVID-19.
【INDEX OF SYMBOL】

【EXPORTER】
Magnus International Limited
F12, New City International Mansion A, 234 Huapao Ave.
Liuyang, Hunan Province 410300 China
Contact: Goodwellmedical@gmail.com
【MANUFACTURER】
Changsha Renji Medical Equipment Co., Ltd.
No.18 Xiangtai Road, Liuyang Jingkai District,
Changsha City, Hunan Province 410300 China
【AUTHORIZED REPRESENTATIVE】
Lotus NL B.V.
Koningin Julianaplein 10, 1e Verd, 2595AA, The Hague, Netherlands.
【FACTORY VIEW】


【FACTORY WORKSHOP】

【PRODUCT AND STANDARDS】

【CERTIFICATIONS】


【FAQ】
1. Q: How about your healthcare and medical product quality?
A: Quality is our top priority. We have a professional quality control team to ensure the
quality with a high standard and regulated with the pro certifications. The QC team is
responsible for quality checking in each process and inspect every batch of our production
and ship them only after QC approval.
2. Q: What is your order MOQ?
A: Please contact the customer service, and the price is negotiable for large quantity.
3. Q: How about your product packing for shipment or mailing?
A: We prepared well neutral package for our products. We could also make OEM package,
OEM/ODM Custom Package/Multi-packing package/Cartons with well protective wrapping.
4. Q: What level of your product price?
A: We are the factory and direct manufacturer and offer competitive prices. We also offer
the volume pricing with more benefits depend on your order quantity level. Please let us know
your ordering volume while you make the inquiry to us to help you obtaining better benefits.
5. Q: What's the price terms?
A: The price is based on FOB of Hunan, China with exchange rate at RMB6.40 for USD$1.00.
If the exchange rate floating sharply lower than 6.3 or higher than 6.5, we should adjust the
price with real-time exchange rate.
6. Q: What's the payment terms?
A: We accept T/T wire transferring, PayPal, Western Union, etc. 50% down payment after
signing the contract and rest 50% before shipping confirmation.
7. Q: How could I get some samples?
A: We are able to deduct your samples expense once your orders confirmed with us.
The shipping fee will be calculated with the sample volume what you demand.
8. Q: Could you accept OEM order?
A: Yes, we could. We have professional designers who can design the logo or artwork and offer
best solution & styles for your OEM product choices.
9. Q: How long will the shipping or express take?
A: (1) For the international expresses such as DHL, UPS, FedEx, TNT, or EMS, etc., it usually
takes 5-7 working days to your location or destination.
(2) If shipping with containers, they will take about 30-60 days to process depending on
the vary ports or door locations.
(3) We will arrange your products delivery or shipping within 1-3 days after full payment
has been confirmed. We may not ship your products out until your payments arrive to
our bank account successfully.
10. Q: How do you deliver the goods to us?
A: If the order of small volume package, we could deliver by courier such as DHL, UPS, FedEx,
TNT, EMS. For the large volume order, we could ship with bulk cargo containers by sea.
We will provide you cost-saving recommendations for your product shipping.
11. Q: What is the warranty time.
A: One year f each product.
【SHIPPING】
We are the trusted healthcare and medical supplies from China.
Be free to contact us at any time!
You are very welcome to email us today at:
Goodwellmedical@gmail.com for the inquiry!
We are excited to build a reliable and long-term business with you
at our best!
![]()